Rational design of de novo proteins for desiccation tolerance
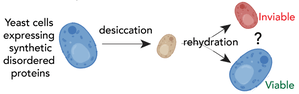
Many intrinsically disordered proteins (IDPs) that confer desiccation tolerance possess transient amphipathic α-helices (TAHs). Transient helices flicker between helical and unstructured states with an average helical occupancy of 30-80%. Amphipathic helices possess two chemically distinct faces: one charged, and one hydrophobic. While TAHs in desiccation were first identified in the context of LEA proteins (a well-studied family of repetitive desiccation-associated proteins), WALII members recently identified TAHs in tardigrade-specific desiccation-protective IDPs (Boothby, et al. 2017; Hesgrove, et al. 2021). TAHs are also found in disordered proteins that do not provide desiccation protection. But what delineates desiccation-protective vs. non-protective TAHs? Here we will take a bottom-up approach and use rational design to systematically uncover the chemical rules that delineate protective vs. non-protective TAHs.
Molecular rules of membraneless organelle water responsiveness

IDPs are often involved in forming biomolecular condensates – non-stoichiometric cellular assemblies. Many condensates play key roles in organismal development and maintenance (e.g., nucleolus, nuclear speckles, P bodies). Given condensates are highly sensitive to changes in their solution environment, changes in water availability can fundamentally alter condensate properties. Despite this, we lack a comprehensive understanding of how condensates change as a function of water. Condensates formed by proteins with different protein sequences have fundamentally distinct properties. As such, a general understanding of condensate water-responsiveness must consider condensates formed from intrinsically disordered regions (IDRs) with different sequence-encoded physicochemical properties based on their amino acid sequence (which we define as sequence chemistry). To address this we will construct synthetic, chemically tunable condensates. We will assess how condensate number, size, material state, and ability to recruit other proteins are altered by changing the chemical environment inside the condensate based on the variable IDR.
Image credit: Steven Boeynaems.


